The fourth state of matter is plasma. Plasma in physics. Plasma in liquids
Return of the sorcerer Koehler Vladimir Romanovich
Plasma - the fourth state of matter
Let's take a metal body, say a bullet, and, putting it in a heat-resistant crucible, put the crucible in an electric furnace. A little time will pass, and the bullet will melt, turn into liquid, and the substance will go into the second state.
But we will turn up the heat. If the furnace's capabilities allow, the metal will eventually boil and evaporate. The substance will go into its third state.
Not so long ago, even the most knowledgeable physicists answered this question that nothing special would happen. The gas will simply heat up more, that's all. Its molecules will acquire high kinetic energy and begin to rush even faster between the walls of the vessel.
There was nothing surprising in this answer. People then did not know how to obtain particularly high temperatures and could not know what would happen to a substance, say, at 6000 degrees. In conventional fuel stoves, the maximum temperature reaches only 2000 degrees, and in electric ones - 3000 degrees.
Now the situation has changed. Even in industrial conditions, temperatures of about 12,000 degrees are sometimes achieved. And physicists have surpassed the limits of the most incredible fantasies in “extracting” high temperatures.
At the Institute of Atomic Energy, researcher M. S. Ioffe carried out experiments in which it was possible to obtain a temperature for deuterium of 60 million degrees - three times higher than in the center of the Sun (according to modern ideas, the temperature in the center of the Sun is slightly less than 20 million degrees ). Academician Evgeniy Konstantinovich Zavoisky achieved even more spectacular results: in his experiments, he and his colleagues managed to heat electron flows to a temperature of over 100 million degrees.
Now it is already known for sure: above 6000 degrees, gases, even those that are stable, seem to evaporate.
What happens to them?
When atoms of a substance collide with one another at breakneck speeds caused by intense heat, electrons are knocked out of them. By losing some electrons, atoms turn into positive ions, that is, into “fragments” charged with positive electricity. Electrons are known to be negatively charged. The result is a mixture of negative electrons, positive ions and neutral atoms that have not had time to “evaporate”. Since the positive electricity in such a mixture is equal to the negative electricity, the mixture as a whole remains neutral. But electrons collide with each other and with ions and make the “evaporated gas” glow (which, however, does not always happen, but only with a sufficient number of particles; if the rarefaction is high, the substance can become completely invisible).
A cloud of matter in this particularly excited state is called plasma. It was discovered in 1920 by the outstanding Indian astrophysicist Meg Nad Saga.
Scientists became convinced quite quickly that plasma is no longer a gas, but a qualitatively completely different, new state of matter.
Each state of matter has its own special properties that are not similar to the properties of other states. Plasma also has them.
The properties of plasma differ sharply from the properties of gas. Gas, for example, is an electrical insulator. Plasma, although it is generally neutral, like a gas, on the contrary, is an excellent conductor of electric current. Unlike metals, which conduct current worse the more they are heated, the electrical conductivity of plasma increases with increasing temperature.
The theory says that at very high temperatures, plasma should practically have the property of superconductivity, that is, its electrical resistance should be close to zero. In addition, plasma is an ideal conductor of heat; it is a superthermal conductive material.
There is a lot of heat in plasma, but there is also something that is not found in any coolant - order. The strong magnetic field in which the plasma is produced introduces an order into its motion, and an unusual one: helical, or otherwise - gyrotropic.
There are many reasons for the keen interest in plasma these days. The first, of course, is that, as it turns out, plasma is much more common in nature than one might think. Almost the entire Universe consists of plasma. The Sun, hot stars, nebulae, and interstellar gas are made of plasma.
It turned out that people had been dealing with plasma long before its discovery.
Water begins to evaporate even before it reaches its boiling point. And plasma is not necessarily formed at a temperature of 6 thousand degrees or above. It occurs, for example, under the influence of strong gas irradiation with X-rays or ultraviolet rays. By placing a gas in a powerful electric field, it can also be brought into a state of ionization and partially converted into plasma.
The candle burns weakly. And yet its flame is, at least to a small extent, ionized. This is not real plasma yet, but already a hint of it. But the dazzling light of an electric arc and the soft glow of a neon tube come directly from the plasma. Close to real plasma is the flame of a welding torch and diesel injector, the flame in the cylinder of an internal combustion engine.
A short-term plasma state occurs in the gun barrel when fired. In general, with any explosion of a large mass of explosive, plasma is formed.
The plasma forms a channel for electric sparks and lightning. The ionized layers in the Earth's atmosphere consist of plasma. The aurora is nothing more than the glow of ionized gas, that is, also plasma.
Yuri Gagarin accomplished his feat literally in the embrace of plasma. When the Vostok spaceship, taking off from the cosmodrome site, pierced the dense layers of the atmosphere with a roar, the nozzles of the rocket engine spewed plasma.
Plasma is widespread everywhere, but perhaps it attracts the attention of scientists even more for its potential for future technology.
Plasma is the most promising state of matter for converting heat directly into electricity. Apparently, in machineless power plants of the future, only plasma will be in motion. Passing between the poles of super-powerful magnets, plasma streams will convert the energy of their movement into the energy of electric current.
The creation of spaceships with plasma engines is not far off. With such engines, emitting a jet plasma jet at speeds of tens or even hundreds of thousands of kilometers per second, you can go to explore the most distant planets of the solar system.
In the spring of 1965, Soviet scientists conducted the first successful tests of plasma engines in space conditions - on board the Zond-2 spacecraft.
Plasma also has great prospects in the field of controlled thermonuclear reactions. Academician L.N. Artsimovich even believes that this is the most important task of plasma. He wrote:
“Plasma physics is not one of the main areas of science, but nevertheless, over the last decade it has been developed very intensively, since hopes are associated with it for solving problems of exceptional promising significance. First place among them is occupied by the well-known problem of controlled thermonuclear fusion, the solution of which should completely eliminate the threat of energy starvation on our planet.”
From the book Medical Physics author Podkolzina Vera Alexandrovna26. Stationary state The principle of entropy production. Organism as an open system The direction of thermodynamic processes in an isolated system was described above. However, real processes and states in nature and technology are nonequilibrium, and many
From the book Return of the Sorcerer author Keler Vladimir RomanovichSolid is the first state of matter. The ancient Greek philosopher Empedocles (490–430 BC) believed that the world was built from four elements, or elements: earth, water, air and fire. The teachings of Empedocles were shared by many ancient scientists, including Aristotle. Then it penetrated
From the book Theory of Relativity for Millions by Gardner MartinLiquid - the second state of matter Remembering the forces acting between the molecules or atoms of solids, it is not difficult to guess why these bodies melt. Because as the temperature increases, the vibrations of each individual atom around its normal position become
From the book Nuclear Energy for Military Purposes author Smith Henry DewolfGaseous - the third state of matter Have you ever wondered which state of matter is most important to us? Almost everyone to whom I asked such a question, asking them to answer without thinking, to answer right away, was mistaken. Then only, in the next moment, they realized:
From the book Hyperspace by Kaku Michio10. Explosion or Steady State Imagine a picture of the gradual expansion of space, and then let this picture go in the opposite direction, as they do in the movies. It is clear that in "the hidden darkness of the past and the abyss of time," as Shakespeare once said, there must have been such
From the book The King's New Mind [On computers, thinking and the laws of physics] by Penrose RogerCURRENT STATE 13.1. As a result of the work of Manhattan District organizations in Washington and Tennessee, groups of scientists in Berkeley, Chicago, Columbia, Los Alamos and other places, industrial groups in Clinton, Hanford and many other places, the end of June 1945 finds us in
From the book Interstellar: the science behind the scenes author Thorne Kip Stephen3. The Man Who “Saw” the Fourth Dimension By 1910, the fourth dimension had become an almost everyday expression... Modified from the ideal Platonic or Kantian reality - or even heaven! - this answer to all the problems puzzling modern science -
From the author's bookThe Fourth Dimension as Art The period from 1890 to 1910 can be considered the golden age of the fourth dimension. It was at this time that the ideas expressed by Gauss and Riemann spread in literary circles, penetrated into the consciousness of the general public, and influenced
From the author's bookThe Bolsheviks and the Fourth Dimension in Tsarist Russia The fourth dimension became famous thanks to the works of the mystic Peter Ouspensky, who introduced Russian intellectuals to the secrets of this dimension. The influence of this topic was felt so clearly that Fedor
From the author's bookBigamists and the Fourth Dimension Eventually the idea of the fourth dimension crossed the Atlantic Ocean to America. Its messenger was a colorful figure - the English mathematician Charles Howard Hinton. If Albert Einstein was poring over his desk in 1905
From the author's bookThe Useless Fourth Dimension In retrospect, Riemann's famous talk was popularized by mystics, philosophers, and artists and made available to a wider audience, but did little to deepen our understanding of nature. Considering
From the author's bookThe Fourth Dimension and Alumni Meetings Of course, Einstein's theory has been presented more than once in popular expositions, the authors of which emphasized different aspects of the theory. But only a few of them grasped the essence of the special theory of relativity: time -
From the author's book From the author's book21. The fourth and fifth dimensions Time as the fourth dimension The space of our Universe has three coordinate axes: “top - bottom”, “east - west” and “north - south”. However, to have lunch with a friend, you will have to agree not only on the meeting place,
From the author's bookTime as the fourth dimension The space of our Universe has three coordinate axes: “top – bottom”, “east – west” and “north – south”. However, to have lunch with a friend, you will have to agree not only on the meeting place, but also on the time. In this sense, time is
From the author's bookChapter 21. The Fourth and Fifth Dimensions For more information on the unification of space and time, see [Thorne 2009]. For John Schwartz and Michael Green's "superstring revolution" and how physicists embraced the multidimensional beam concept, see The Elegant Universe. Superstrings, hidden
In addition to the three states listed above, a substance can be in a fourth state of aggregation - plasma , which was discovered relatively recently. The plasma state occurs when a substance in a gaseous state is exposed to such strong ionizing factors as ultra-high temperatures (several million degrees), powerful electrical discharges or electromagnetic radiation. In this case, the molecules and atoms of the substance are destroyed and transformed into a mixture consisting of positively charged nuclei and electrons moving at colossal speeds. For this reason, plasma is sometimes called an electron-nuclear gas.
There are two types of plasma: isothermal and gas-discharge.
Isothermal plasma It is obtained at high temperatures, under the influence of which thermal dissociation of the atoms of the substance takes place, and can exist indefinitely. This type of plasma is the substance of stars, as well as ball lightning. The Earth's ionosphere is also a special type of plasma; however, in this case, ionization occurs under the influence of ultraviolet radiation from the Sun.
Isothermal plasma plays an extremely important role in space processes. Three other aggregate states of matter in outer space are exceptions.
Gas discharge plasma is formed during an electrical discharge and is therefore stable only in the presence of an electric field. As soon as the action of the external field ceases, the gas-discharge plasma, due to the formation of neutral atoms from ions and electrons, disappears within 10 –5 -10 –4 s.
One of the remarkable properties of plasma is its high electrical conductivity. The higher the temperature of the plasma, the higher its conductivity. Because of this, currents of hundreds of thousands and millions of amperes can be passed through plasma.
By passing such currents through a plasma, it is possible to raise its temperature to tens and even hundreds of millions of degrees, and its pressure to tens of gigapascals. Such conditions are known to be close to holding thermonuclear fusion reactions , which can produce colossal amounts of energy.
As is known, energy is released not only during the fission of nuclei, but also during their fusion, i.e., during the fusion of lighter nuclei into heavier ones. The task in this case is to overcome electrical repulsion and bring light nuclei closer to sufficiently small distances where nuclear attractive forces begin to act between them. So, for example, if it were possible to force two protons and two neutrons to combine into the nucleus of a helium atom, then enormous energy would be released. By heating to high temperatures as a result of ordinary collisions, nuclei can approach such small distances that nuclear forces come into play and fusion occurs. Once started, the fusion process, as calculations show, can provide the amount of heat needed to maintain the high temperature necessary for further nuclear fusions, i.e. the process will continue continuously. This produces such a powerful source of thermal energy that its amount can be controlled only by the amount of material required. This is the essence of conducting a controlled thermonuclear fusion reaction.
When an electric current passes through a plasma, it creates a strong magnetic field that compresses the flow of electrons and ions into plasma cord This achieves thermal insulation of the plasma from the walls of the vessel. As the current increases, the electromagnetic compression of the plasma becomes more pronounced. This is the essence of the so-called pinch effect .As research has shown, the pinch effect and the forces created by external magnetic fields varying according to a certain law can be successfully used to hold plasma in a “magnetic bottle” where the fusion reaction occurs.
CHEMICAL BOND THEORY
General provisions of the doctrine of chemical bonds. Covalent bond
The concept of a chemical bond is one of the fundamental ones in modern science. Without knowledge of the nature of the interaction of atoms, it is impossible to understand the mechanism of formation of chemical compounds, their composition and reactivity, and even more so, to predict the properties of new materials.
The very first and not entirely clear ideas about chemical bonds were introduced by Kekule in 1857. He pointed out that the number of atoms bonded to an atom of another element depends on the basicity of the constituent parts .
For the first time, the term “chemical bond” was introduced by A.M. Butlerov in 1863. In the creation of the doctrine of chemical bonds, his theory of chemical structure, proposed in 1861, played a large role. However, having formulated the main provisions of the theory, Butlerov did not yet use the term “chemical bond”. The tenets of his teaching are as follows:
1. Atoms in molecules are connected to each other in a certain sequence. Changing this sequence leads to the formation of a new substance with new properties.
2. The connection of atoms occurs in accordance with their valency.
3. The properties of substances depend not only on the composition, but also on their “chemical structure”, i.e. on the order of connection of atoms in molecules and the nature of their mutual influence.
Thus, the properties of substances are determined not only by their qualitative and quantitative composition, but also by the internal structure of the molecules.
In 1863, in his work “On various explanations of some cases of isomerism,” Butlerov already spoke about “the method of chemical bonding between atoms,” about “the chemical bonding of individual atoms.”
What does the term “chemical bond” mean?
A number of definitions of this concept can be given, but the most obvious of them is that chemical bond – this is the interaction that occurs between atoms during the formation of substances.
A scientific explanation of the nature of the chemical bond could appear only after the emergence of the doctrine of the structure of the atom. In 1916, the American physical chemist Lewis suggested that a chemical bond arises by pairing electrons belonging to different atoms. This idea was the starting point for modern theory of covalent chemical bond .
In the same year, the German scientist Kossel suggested that when two atoms interact, one of them gives away and the other accepts electrons. The electrostatic interaction of the resulting ions leads to the formation of a stable compound. The development of Kossel's ideas led to the creation ionic bond theory .
In any case, the chemical bond is of electrical origin, because is ultimately due to the interaction of electrons.
One of the reasons for the emergence of a chemical bond is the desire of atoms to assume a more stable state. A necessary condition for the formation of a chemical bond is a decrease in the potential energy of a system of interacting atoms.
During chemical reactions, the nuclei of atoms and internal electron shells do not undergo changes. Chemical bonding occurs through the interaction of the electrons most distant from the nucleus, called valence .
Valence elements are: for s-elements - s-electrons of the outer energy level, for p-elements - s- and p-electrons of the outer energy level, for d-elements - s-electrons of the outer and d-electrons of the pre-external energy levels, for f- elements - s-electrons of the outer and f-electrons of the third outside energy levels.
There are usually five main types of chemical bonding: ionic, covalent, metallic, hydrogen, and intermolecular interactions , caused by van der Waals forces, and the first three types of connection are significantly stronger than the last two.
The modern doctrine of chemical bonding is based on quantum mechanical concepts. Two methods are currently widely used to describe chemical bonds: valence bond method(MVS) and molecular orbital method(MMO).
The BC method is simpler and more visual, so we will begin our consideration of the theory of chemical bonding with it.
Let's consider the most common covalent chemical bond.
Valence bond method
The BC method is based on the following provisions.
1. A covalent chemical bond is formed by two electrons with opposite spins, and this electron pair belongs simultaneously to two atoms. The atoms themselves retain their individuality.
2. A covalent chemical bond is stronger the more the interacting electron clouds overlap.
In the broad sense of the word covalent bond is a chemical bond between atoms carried out by sharing electrons. A covalent bond can be considered as a universal, most common type of chemical bond.
To accurately describe the state of an electron in a molecule, it is necessary to solve the Schrödinger equation for the corresponding system of electrons and nuclei, specifying the condition of minimum energy. However, at present, solving the Schrödinger equation is possible only for the simplest systems. The first approximate calculation of the electron wave function was made in 1927 by Heitler and London for the hydrogen molecule.
Rice. 4.1. Dependence of the energy of a system of two hydrogen atoms on
internuclear distance for electrons with parallel (1) and
antiparallel (2) spins.
As a result of their work, they obtained an equation relating the potential energy of the system to the distance between the nuclei of two hydrogen atoms. It turned out that the calculation results depend on whether the spins of both electrons are the same or opposite in sign.
With parallel spins, the approach of atoms leads to a continuous increase in the energy of the system. With oppositely directed spins, atoms approach each other to a certain distance r 0 is accompanied by a decrease in the energy of the system, after which it begins to increase again (Fig. 4.1).
Thus, if the electron spins are parallel, the formation of a chemical bond does not occur for energy reasons, but in the case of oppositely directed electron spins, an H2 molecule is formed - a stable system of two hydrogen atoms, the distance between the nuclei of which is r 0 .
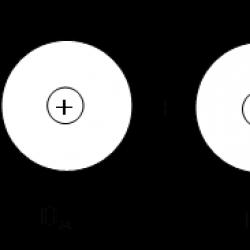
This is the distance r 0 significantly less than twice the atomic radius (for a hydrogen molecule - 0.074 and 0.106 nm, respectively), therefore, when a chemical bond is formed, mutual overlap of electron clouds and reacting atoms occurs (Fig. 3.2).
 |
Rice. 4.2. Scheme of electron cloud overlap during formation
hydrogen molecules
Due to the overlapping of the clouds, the electron density between the nuclei increases, and the attractive forces between this region of negative charge and the positively charged nuclei of interacting atoms increase. An increase in attractive forces is accompanied by the release of energy, which leads to the formation of a chemical bond.
When depicting structural formulas, a bond is indicated by a dash or two dots (a dot denotes an electron):
N – N N: N
In the case considered, electrons located in the s-orbitals of hydrogen atoms are shared. The hydrogen atom has no other electrons. In the case of, for example, halogens, each interacting atom also has three pairs of electrons at the external energy level that are not involved in the formation of a chemical bond (two s-electrons and four p-electrons):
 |
The chemical bond in the F2 molecule is formed due to the interaction of unpaired electrons located in atomic p-orbitals; the remaining electrons do not take part in the formation of the chemical bond (they are often called lone electron pairs).
Only one electron from each atom takes part in the formation of H 2 and F 2 molecules. A covalent bond formed by one pair of electrons is called single communication
A bond formed by two or three pairs of electrons is called multiple communication Thus, oxygen and nitrogen atoms contain two and three unpaired electrons, respectively:
 |
Consequently, two or three electrons from each atom, respectively, take part in the formation of O 2 and N 2 molecules. Thus, the bond in the oxygen molecule is double, and in the nitrogen molecule it is triple:
How can a multiple bond be formed? Are all connections equal in these cases? To answer this and other related questions, we should consider the basic characteristics of a covalent bond.
What is the fourth state of matter, how does it differ from the other three and how to make it serve a person.
The assumption of the existence of the first of the states of matter beyond the classical triad was made at the beginning of the 19th century, and in the 1920s it received its name - plasma
Alexey Levin
A hundred and fifty years ago, almost all chemists and many physicists believed that matter consists only of atoms and molecules that are combined into more or less ordered or completely disordered combinations. Few doubted that all or almost all substances are capable of existing in three different phases - solid, liquid and gaseous, which they take on depending on external conditions. But hypotheses about the possibility of other states of matter have already been expressed.
This universal model was confirmed by both scientific observations and millennia of experience in everyday life. After all, everyone knows that when water cools, it turns into ice, and when heated, it boils and evaporates. Lead and iron can also be converted into liquid and gas, they just need to be heated more strongly. Since the late 18th century, researchers had been freezing gases into liquids, and it seemed plausible that any liquefied gas could in principle be made to solidify. In general, a simple and understandable picture of the three states of matter seemed to require no corrections or additions.

Scientists of that time would have been quite surprised to learn that the solid, liquid and gaseous states of atomic-molecular matter are preserved only at relatively low temperatures, not exceeding 10,000°, and even in this zone they do not exhaust all possible structures (for example, liquid crystals). It would not be easy to believe that they account for no more than 0.01% of the total mass of the current Universe. Now we know that matter realizes itself in many exotic forms. Some of them (such as degenerate electron gas and neutron matter) exist only inside super-dense cosmic bodies (white dwarfs and neutron stars), and some (such as quark-gluon liquid) were born and disappeared in a brief moment shortly after the Big Bang. However, it is interesting that the assumption about the existence of the first of the states that go beyond the classical triad was made in the same nineteenth century, and at its very beginning. It became a subject of scientific research much later, in the 1920s. That’s when it got its name—plasma.
From Faraday to Langmuir
In the second half of the 70s of the 19th century, William Crookes, a member of the Royal Society of London, a very successful meteorologist and chemist (he discovered thallium and extremely accurately determined its atomic weight), became interested in gas discharges in vacuum tubes. By that time it was known that the negative electrode emits emanations of an unknown nature, which the German physicist Eugen Goldstein in 1876 called cathode rays. After many experiments, Crookes decided that these rays were nothing more than gas particles, which, after colliding with the cathode, acquired a negative charge and began to move towards the anode. He called these charged particles “radiant matter”.

It should be admitted that Crookes was not original in this explanation of the nature of cathode rays. Back in 1871, a similar hypothesis was expressed by the prominent British electrical engineer Cromwell Fleetwood Varley, one of the leaders of the work on laying the first transatlantic telegraph cable. However, the results of experiments with cathode rays led Crookes to a very deep thought: the medium in which they propagate is no longer a gas, but something completely different. On August 22, 1879, at a session of the British Association for the Advancement of Science, Crookes declared that discharges in rarefied gases “are so unlike anything that happens in air or any gas under ordinary pressure, that in this case we are dealing with a substance in the fourth state, which in properties differs from ordinary gas to the same extent as a gas differs from a liquid.”
It is often written that it was Crookes who first thought of the fourth state of matter. In fact, this idea occurred to Michael Faraday much earlier. In 1819, 60 years before Crookes, Faraday proposed that matter could exist in solid, liquid, gaseous and radiant states, the radiant state of matter. In his report, Crookes directly said that he was using terms borrowed from Faraday, but for some reason his descendants forgot about this. However, Faraday's idea was still a speculative hypothesis, and Crookes substantiated it with experimental data.
Cathode rays were intensively studied even after Crookes. In 1895, these experiments led William Roentgen to the discovery of a new type of electromagnetic radiation, and at the beginning of the twentieth century resulted in the invention of the first radio tubes. But Crookes's hypothesis of a fourth state of matter did not attract interest among physicists, most likely because in 1897 Joseph John Thomson proved that cathode rays were not charged gas atoms, but very light particles, which he called electrons. This discovery seemed to render Crookes's hypothesis unnecessary.

However, she was reborn like a phoenix from the ashes. In the second half of the 1920s, the future Nobel laureate in chemistry Irving Langmuir, who worked in the laboratory of the General Electric Corporation, began to study gas discharges in earnest. Then they already knew that in the space between the anode and cathode, gas atoms lose electrons and turn into positively charged ions. Realizing that such a gas had many special properties, Langmuir decided to give it his own name. By some strange association, he chose the word “plasma,” which had previously been used only in mineralogy (this is another name for green chalcedony) and in biology (the liquid basis of blood, as well as whey). In its new capacity, the term “plasma” first appeared in Langmuir’s article “Oscillations in Ionized Gases,” published in 1928. For about thirty years, few people used this term, but then it firmly entered into scientific use.
Plasma physics
Classical plasma is an ion-electron gas, possibly diluted with neutral particles (strictly speaking, photons are always present there, but at moderate temperatures they can be ignored). If the degree of ionization is not too low (usually one percent is enough), this gas exhibits many specific qualities that ordinary gases do not possess. However, it is possible to produce a plasma in which there will be no free electrons at all, and negative ions will take on their responsibilities.

For simplicity, we will consider only electron-ion plasma. Its particles are attracted or repelled in accordance with Coulomb's law, and this interaction manifests itself over large distances. This is precisely why they differ from atoms and molecules of neutral gas, which feel each other only at very short distances. Since plasma particles are in free flight, they are easily displaced by electrical forces. In order for the plasma to be in a state of equilibrium, it is necessary that the space charges of electrons and ions completely compensate each other. If this condition is not met, electric currents arise in the plasma, which restore equilibrium (for example, if an excess of positive ions is formed in some area, electrons will instantly rush there). Therefore, in an equilibrium plasma, the densities of particles of different signs are practically the same. This most important property is called quasineutrality.
Almost always, atoms or molecules of an ordinary gas participate only in pair interactions - they collide with each other and fly apart. Plasma is a different matter. Since its particles are connected by long-range Coulomb forces, each of them is in the field of near and distant neighbors. This means that the interaction between plasma particles is not paired, but multiple—as physicists say, collective. This leads to the standard definition of plasma - a quasi-neutral system of a large number of unlike charged particles exhibiting collective behavior.

Plasma differs from neutral gas in its reaction to external electric and magnetic fields (ordinary gas practically does not notice them). Plasma particles, on the contrary, sense arbitrarily weak fields and immediately begin to move, generating space charges and electric currents. Another important feature of equilibrium plasma is charge shielding. Let's take a plasma particle, say a positive ion. It attracts electrons, which form a cloud of negative charge. The field of such an ion behaves in accordance with Coulomb's law only in its vicinity, and at distances exceeding a certain critical value it very quickly tends to zero. This parameter is called the Debye screening radius, after the Dutch physicist Pieter Debye, who described this mechanism in 1923.
It is easy to understand that plasma retains quasineutrality only if its linear dimensions in all dimensions greatly exceed the Debye radius. It is worth noting that this parameter increases when the plasma is heated and decreases as its density increases. In the plasma of gas discharges, the order of magnitude is 0.1 mm, in the earth's ionosphere - 1 mm, in the solar core - 0.01 nm.
Controlled thermonuclear
Plasma is used in a wide variety of technologies these days. Some of them are known to everyone (gas light lamps, plasma displays), others are of interest to specialized specialists (production of heavy-duty protective film coatings, production of microchips, disinfection). However, the greatest hopes for plasma are placed in connection with work on the implementation of controlled thermonuclear reactions. This is understandable. In order for hydrogen nuclei to merge into helium nuclei, they must be brought together to a distance of about one hundred billionth of a centimeter - and then nuclear forces will begin to work. Such a rapprochement is possible only at temperatures of tens and hundreds of millions of degrees - in this case, the kinetic energy of positively charged nuclei is enough to overcome electrostatic repulsion. Therefore, controlled thermonuclear fusion requires high-temperature hydrogen plasma.

True, plasma based on ordinary hydrogen will not help here. Such reactions occur in the depths of stars, but they are useless for terrestrial energy because the intensity of energy release is too low. It is best to use plasma from a mixture of the heavy hydrogen isotopes deuterium and tritium in a 1:1 ratio (pure deuterium plasma is also acceptable, although it will provide less energy and require higher temperatures for ignition).
However, heating alone is not enough to start the reaction. Firstly, the plasma must be sufficiently dense; secondly, particles entering the reaction zone should not leave it too quickly - otherwise the loss of energy will exceed its release. These requirements can be presented in the form of a criterion that was proposed by the English physicist John Lawson in 1955. According to this formula, the product of the plasma density and the average particle confinement time must be higher than a certain value determined by the temperature, the composition of the thermonuclear fuel and the expected efficiency of the reactor.

It is easy to see that there are two ways to satisfy Lawson's criterion. It is possible to reduce the confinement time to nanoseconds by compressing the plasma, say, to 100−200 g/cm3 (since the plasma does not have time to fly apart, this confinement method is called inertial). Physicists have been working on this strategy since the mid-1960s; Now its most advanced version is being developed by the Livermore National Laboratory. This year, they will begin experiments on compressing miniature beryllium capsules (diameter 1.8 mm), filled with a deuterium-tritium mixture, using 192 ultraviolet laser beams. Project leaders believe that no later than 2012 they will be able not only to ignite a thermonuclear reaction, but also to obtain a positive energy output. Perhaps a similar program within the HiPER (High Power Laser Energy Research) project will be launched in Europe in the coming years. However, even if the experiments at Livermore fully live up to their expectations, the distance to the creation of a real thermonuclear reactor with inertial plasma confinement will still remain very large. The fact is that to create a prototype power plant, a very fast-firing system of super-powerful lasers is needed. It should provide a frequency of flashes that ignite deuterium-tritium targets that will be thousands of times greater than the capabilities of the Livermore system, which fires no more than 5-10 shots per second. Various possibilities for creating such laser guns are now being actively discussed, but their practical implementation is still very far away.
Tokamaki: the old guard
Alternatively, one can work with a rarefied plasma (density of nanograms per cubic centimeter), holding it in the reaction zone for at least a few seconds. In such experiments, for more than half a century, various magnetic traps have been used, which keep plasma in a given volume by applying several magnetic fields. The most promising are considered tokamaks - closed magnetic traps in the shape of a torus, first proposed by A.D. Sakharov and I.E. Tamm in 1950. Currently, there are a dozen such installations operating in various countries, the largest of which have brought them closer to meeting the Lawson criterion. The international experimental thermonuclear reactor, the famous ITER, which will be built in the village of Cadarache near the French city of Aix-en-Provence, is also a tokamak. If all goes according to plan, ITER will make it possible for the first time to produce plasma that satisfies the Lawson criterion and ignite a thermonuclear reaction in it.

“Over the past two decades, we have made enormous progress in understanding the processes that occur inside magnetic plasma traps, in particular tokamaks. In general, we already know how plasma particles move, how unstable states of plasma flows arise, and to what extent the plasma pressure can be increased so that it can still be contained by a magnetic field. New high-precision methods of plasma diagnostics have also been created, that is, measuring various plasma parameters,” Ian Hutchinson, a professor of nuclear physics and nuclear technology at the Massachusetts Institute of Technology, who has been working on tokamaks for over 30 years, told PM. — To date, the largest tokamaks have achieved thermal energy release powers in deuterium-tritium plasma of the order of 10 megawatts for one to two seconds. ITER will exceed these figures by a couple of orders of magnitude. If we are not mistaken in our calculations, it will be able to produce at least 500 megawatts within a few minutes. If you’re really lucky, energy will be generated without any time limit at all, in a stable mode.”
Professor Hutchinson also emphasized that scientists now have a good understanding of the nature of the processes that must occur inside this huge tokamak: “We even know the conditions under which the plasma suppresses its own turbulence, and this is very important for controlling the operation of the reactor. Of course, it is necessary to solve many technical problems - in particular, to complete the development of materials for the internal lining of the chamber that can withstand intense neutron bombardment. But from the point of view of plasma physics, the picture is quite clear - at least we think so. ITER must confirm that we are not mistaken. If everything goes well, the turn of the next generation tokamak will come, which will become a prototype of industrial thermonuclear reactors. But now it’s too early to talk about it. In the meantime, we expect ITER to become operational by the end of this decade. Most likely, it will be able to generate hot plasma no earlier than 2018, at least according to our expectations.” So from the point of view of science and technology, the ITER project has good prospects.
I think everyone knows the 3 main states of matter: liquid, solid and gaseous. We encounter these states of matter every day and everywhere. Most often they are considered using the example of water. The liquid state of water is most familiar to us. We constantly drink liquid water, it flows from our tap, and we ourselves are 70% liquid water. The second physical state of water is ordinary ice, which we see on the street in winter. Water is also easy to find in gaseous form in everyday life. In the gaseous state, water is, as we all know, steam. It can be seen when, for example, we boil a kettle. Yes, it is at 100 degrees that water changes from liquid to gaseous.
These are the three states of matter that are familiar to us. But did you know that there are actually 4 of them? I think everyone has heard the word “plasma” at least once. And today I want you to also learn more about plasma - the fourth state of matter.
Plasma is a partially or fully ionized gas with equal densities of both positive and negative charges. Plasma can be obtained from gas - from the 3rd state of aggregation of a substance by strong heating. The state of aggregation in general, in fact, completely depends on temperature. The first state of aggregation is the lowest temperature at which the body remains solid, the second state of aggregation is the temperature at which the body begins to melt and become liquid, the third state of aggregation is the highest temperature, at which the substance becomes a gas. For each body, substance, the temperature of transition from one state of aggregation to another is completely different, for some it is lower, for some it is higher, but for everyone it is strictly in this sequence. At what temperature does a substance become plasma? Since this is the fourth state, it means that the temperature of transition to it is higher than that of each previous one. And indeed it is. In order to ionize a gas, a very high temperature is required. The lowest temperature and low ionized (about 1%) plasma is characterized by a temperature of up to 100 thousand degrees. Under terrestrial conditions, such plasma can be observed in the form of lightning. The temperature of the lightning channel can exceed 30 thousand degrees, which is 6 times higher than the temperature of the surface of the Sun. By the way, the Sun and all other stars are also plasma, most often high-temperature. Science proves that about 99% of all matter in the Universe is plasma.
Unlike low-temperature plasma, high-temperature plasma has almost 100% ionization and a temperature of up to 100 million degrees. This is truly a stellar temperature. On Earth, such plasma is found only in one case - for thermonuclear fusion experiments. A controlled reaction is quite complex and energy-consuming, but an uncontrolled reaction has proven itself to be a weapon of colossal power - a thermonuclear bomb tested by the USSR on August 12, 1953.
Plasma is classified not only by temperature and degree of ionization, but also by density and quasi-neutrality. Collocation plasma density usually means electron density, that is, the number of free electrons per unit volume. Well, with this, I think everything is clear. But not everyone knows what quasi-neutrality is. Plasma quasineutrality is one of its most important properties, which consists in the almost exact equality of the densities of the positive ions and electrons included in its composition. Due to the good electrical conductivity of plasma, the separation of positive and negative charges is impossible at distances greater than the Debye length and at times greater than the period of plasma oscillations. Almost all plasma is quasi-neutral. An example of a non-quasi-neutral plasma is an electron beam. However, the density of non-neutral plasmas must be very small, otherwise they will quickly decay due to Coulomb repulsion.
We have looked at very few terrestrial examples of plasma. But there are quite a lot of them. Man has learned to use plasma for his own benefit. Thanks to the fourth state of matter, we can use gas-discharge lamps, plasma TVs, electric arc welding, and lasers. Conventional fluorescent discharge lamps are also plasma. There is also a plasma lamp in our world. It is mainly used in science to study and, most importantly, see some of the most complex plasma phenomena, including filamentation. A photograph of such a lamp can be seen in the picture below:
In addition to household plasma devices, natural plasma can also often be seen on Earth. We have already talked about one of her examples. This is lightning. But in addition to lightning, plasma phenomena can be called the northern lights, “St. Elmo’s fire,” the Earth’s ionosphere and, of course, fire.

Notice that fire, lightning, and other manifestations of plasma, as we call it, burn. What causes such a bright light emission from plasma? Plasma glow is caused by the transition of electrons from a high-energy state to a low-energy state after recombination with ions. This process results in radiation with a spectrum corresponding to the excited gas. This is why plasma glows.
I would also like to talk a little about the history of plasma. After all, once upon a time only such substances as the liquid component of milk and the colorless component of blood were called plasma. Everything changed in 1879. It was in that year that the famous English scientist William Crookes, while studying electrical conductivity in gases, discovered the phenomenon of plasma. True, this state of matter was called plasma only in 1928. And this was done by Irving Langmuir.
In conclusion, I want to say that such an interesting and mysterious phenomenon as ball lightning, which I have written about more than once on this site, is, of course, also a plasmoid, like ordinary lightning. This is perhaps the most unusual plasmoid of all terrestrial plasma phenomena. After all, there are about 400 different theories about ball lightning, but not one of them has been recognized as truly correct. In laboratory conditions, similar but short-term phenomena were obtained in several different ways, so the question of the nature of ball lightning remains open.
Ordinary plasma, of course, was also created in laboratories. This was once difficult, but now such an experiment is not particularly difficult. Since plasma has firmly entered our everyday arsenal, they are experimenting a lot on it in laboratories.
The most interesting discovery in the field of plasma was experiments with plasma in zero gravity. It turns out that plasma crystallizes in a vacuum. It happens like this: charged plasma particles begin to repel each other, and when they have a limited volume, they occupy the space that is allotted to them, scattering in different directions. This is quite similar to a crystal lattice. Doesn't this mean that plasma is the closing link between the first state of matter and the third? After all, it becomes plasma due to the ionization of the gas, and in a vacuum the plasma again becomes solid. But this is just my guess.
Plasma crystals in space also have a rather strange structure. This structure can only be observed and studied in space, in the real vacuum of space. Even if you create a vacuum on Earth and place plasma there, gravity will simply compress the entire “picture” that forms inside. In space, plasma crystals simply take off, forming a three-dimensional three-dimensional structure of a strange shape. After sending the results of observing plasma in orbit to scientists on Earth, it turned out that the vortices in the plasma strangely repeat the structure of our galaxy. This means that in the future it will be possible to understand how our galaxy was born by studying plasma. The photographs below show the same crystallized plasma.
Typical plasma examples
Plasma is the most common state of matter. More than 99% of what is observed consists of plasma. The following forms of plasma are well known:
- Laboratory and industrial
- Flames
- Welding arc
- Rocket exhaust
- Plasma for controlled thermonuclear fusion
- Natural
- and others (formed by thermonuclear fusion)
- Interstellar gas
Properties
The term plasma is used for systems of charged particles large enough to produce collective effects. Microscopic small amounts of charged particles (eg ion beams in ion traps) are not plasma. Plasma has the following properties:
- The Debye screening length is small compared to the characteristic size of the plasma.
- r_D/L<<1\,
- Inside the sphere c there is a large number of charged particles.
- r_D^3N>>1\,, Where N\,- concentration of charged particles
- The average time between particle collisions is long compared to the period of plasma oscillations.
- \tau\omega_(pl)>>1\,
Classification
Plasma is usually divided into low temperature And high temperature, equilibrium And nonequilibrium, and quite often cold plasma is nonequilibrium, and hot plasma is equilibrium.
Temperature
In nonequilibrium plasmas, the electron temperature significantly exceeds the ion temperature. This occurs due to the difference in the masses of the ion and electron, which makes the process of energy exchange difficult. This situation occurs in gas discharges, when the ions have a temperature of about hundreds, and the electrons have a temperature of about tens of thousands of degrees.
In equilibrium plasmas both temperatures are equal. Since the ionization process requires temperatures comparable to the ionization potential, equilibrium plasmas are usually hot (with temperatures greater than several thousand degrees).
Concept high temperature plasma usually used for thermonuclear fusion plasma, which requires temperatures of millions of degrees.
Degree of ionization
The degree of ionization is defined as the ratio of the number of ionized particles to the total number of particles. Low-temperature plasmas are characterized by low degrees of ionization (<1 %). Так как такие плазмы довольно часто употребляются в plasma technologies they are sometimes called technological plasmas. Most often, they are created using electric fields that accelerate electrons, which in turn ionize atoms. Electric fields are introduced into the gas through inductive or capacitive coupling. Typical applications of low-temperature plasmas include plasma modification of surface properties (diamond films, metal nitridation, wettability modification), plasma etching of surfaces (semiconductor industry), purification of gases and liquids (ozonation of water and combustion of soot particles in diesel engines).
Hot plasmas almost always completely ionized (ionization degree ~100%). Usually they are understood as the “fourth state of matter.” An example is the Sun.
Density
Besides temperature, which is fundamental to the very existence of a plasma, the second most important property of a plasma is its density. Word plasma density usually means electron density, that is, the number of free electrons per unit volume (strictly speaking, here, density is called concentration - not the mass of a unit volume, but the number of particles per unit volume). Ion density connected to it through the average charge number of ions \langle Z\rangle: n_e=\langle Z\rangle n_i. The next important quantity is the density of neutral atoms n 0 . In hot plasma n 0 is small, but may nevertheless be important for the physics of processes in plasma.
Quasi-neutrality
Since plasma is a very good conductor, electrical properties are important. Plasma potential or potential of space is called the average value of the electric potential at a given point in space. If any body is introduced into the plasma, its potential will generally be less than the plasma potential due to the appearance of the Debye layer. This potential is called floating potential. Due to its good electrical conductivity, plasma tends to shield all electric fields. This leads to the phenomenon of quasineutrality - the density of negative charges is equal to the density of positive charges with good accuracy ( n_e=\langle Z\rangle n_i). Due to the good electrical conductivity of plasma, the separation of positive and negative charges is impossible at distances greater than the Debye length and at times greater than the period of plasma oscillations.
An example of a non-quasi-neutral plasma is an electron beam. However, the density of non-neutral plasmas must be very small, otherwise they will quickly decay due to Coulomb repulsion.
Differences from the gaseous state
Plasma is often called fourth state of matter. It differs from the three less energetic states of matter, although it is similar to the gas phase in that it does not have a specific shape or volume. There is still debate about whether plasma is a separate state of aggregation, or simply a hot gas. Most physicists believe that plasma is more than a gas due to the following differences:
| Property | Gas | Plasma |
| Electrical conductivity | Very small |
Very high
|
| Number of particle types | One | Two or three Electrons, ions and neutral particles are distinguished by their electron sign. charge and can behave independently of each other - have different speeds and even temperatures, which causes the appearance of new phenomena, such as waves and instabilities. |
| Speed distribution | Maxwell's | May be non-Maxwellian Electric fields have a different effect on particle velocities than collisions, which always lead to a Maxwellization of the velocity distribution. The velocity dependence of the Coulomb collision cross section can enhance this difference, leading to effects such as two-temperature distributions and runaway electrons. |
| Type of interactions | Binary As a rule, two-particle collisions, three-particle collisions are extremely rare. |
Collective Each particle interacts with many at once. These collective interactions have a much greater impact than two-particle interactions. |
Mathematical description
Plasma can be described at various levels of detail. Usually plasma is described separately from electromagnetic fields. A joint description of a conducting fluid and electromagnetic fields is given in the theory of magnetohydrodynamic phenomena or MHD theory.
Fluid (liquid) model
In the fluid model, electrons are described in terms of density, temperature, and average velocity. The model is based on: the balance equation for density, the momentum conservation equation, and the electron energy balance equation. In the two-fluid model, ions are treated in the same way.
Kinetic description
Sometimes the liquid model is not sufficient to describe plasma. A more detailed description is given by the kinetic model. Plasma is described in terms of the Electron Velocity Distribution Function. The model is based on. When describing plasma and electricity together. fields, the Vlasov system of equations is used. The kinetic description must be used in the absence of thermodynamic equilibrium or in the presence of strong plasma inhomogeneities.
Particle-In-Cell (particle in a cell)
Particle-In-Cell models are more detailed than kinetic models. They incorporate kinetic information by tracking the trajectories of large numbers of individual particles. El. Density charge and current are determined by summing particles in cells that are small compared to the problem under consideration but nevertheless contain a large number of particles. Email and mag. The fields are found from the charge and current densities at the cell boundaries.
Basic plasma characteristics
All quantities are given in Gaussian units except temperature, which is given in eV and ion mass, which is given in proton mass units. μ = m i / m p ; Z– charge number; k– Boltzmann constant; TO– wavelength; γ - adiabatic index; ln Λ - Coulomb logarithm.
Frequencies
- Larmor frequency of electron, angular frequency of the electron’s circular motion in a plane perpendicular to the magnetic field:
- Larmor frequency of the ion, angular frequency of the circular motion of the ion in a plane perpendicular to the magnetic field:
- plasma frequency(plasma oscillation frequency), the frequency with which electrons oscillate around the equilibrium position when displaced relative to the ions:
- ion plasma frequency:
- electron collision frequency
- ion collision frequency
Lengths
- De Broglie electron wavelength, electron wavelength in quantum mechanics:
- minimum approach distance in the classical case, the minimum distance to which two charged particles can approach in a head-on collision and an initial speed corresponding to the temperature of the particles, neglecting quantum mechanical effects:
- electron gyromagnetic radius, radius of circular motion of an electron in a plane perpendicular to the magnetic field:
r_e = v_(Te)/\omega_(ce) = 2.38\,T_e^(1/2)B^(-1)\,\mbox(cm)
- ion gyromagnetic radius, radius of circular motion of the ion in a plane perpendicular to the magnetic field:
- plasma skin layer size, the distance at which electromagnetic waves can penetrate the plasma:
- (Debye length), the distance at which electric fields are screened due to the redistribution of electrons:
Speeds
- thermal electron velocity, formula for estimating the speed of electrons at . Average speed, most probable speed and root mean square speed differ from this expression only by factors of the order of unity:
- thermal ion velocity, formula for estimating the ion velocity at